LOUISVILLE, Ky. – Research in the past two decades has revealed that microbial organisms in the gut influence health and disease in many ways, particularly related to immune function, metabolism and resistance to infection. Recent studies have shown that gut microbes also may cause or worsen Parkinson’s disease, Alzheimer’s disease and other neurodegenerative conditions.
University of Louisville neurology professor Robert P. Friedland, M.D., and Matthew R. Chapman, Ph.D., professor at the University of Michigan, have proposed a new term to describe an interaction between gut microbiota and the brain in an article released today in PLOS Pathogens.
Friedland and Chapman propose the term “mapranosis” for the process by which amyloid proteins produced by microbes (bacteria, fungi and others) alter the structure of proteins (proteopathy) and enhance inflammation in the nervous system, thereby initiating or augmenting brain disease. The term is derived from Microbiota Associated Protepathy And Neuroinflammation + osis (a process).
Friedland hopes that giving the process a name will facilitate awareness of the process, as well as research leading to therapeutic opportunities.
“It is critical to define the ways in which gut bacteria and other organisms interact with the host to create disease, as there are many ways in which the microbiota may be altered to influence health,” Friedland said.
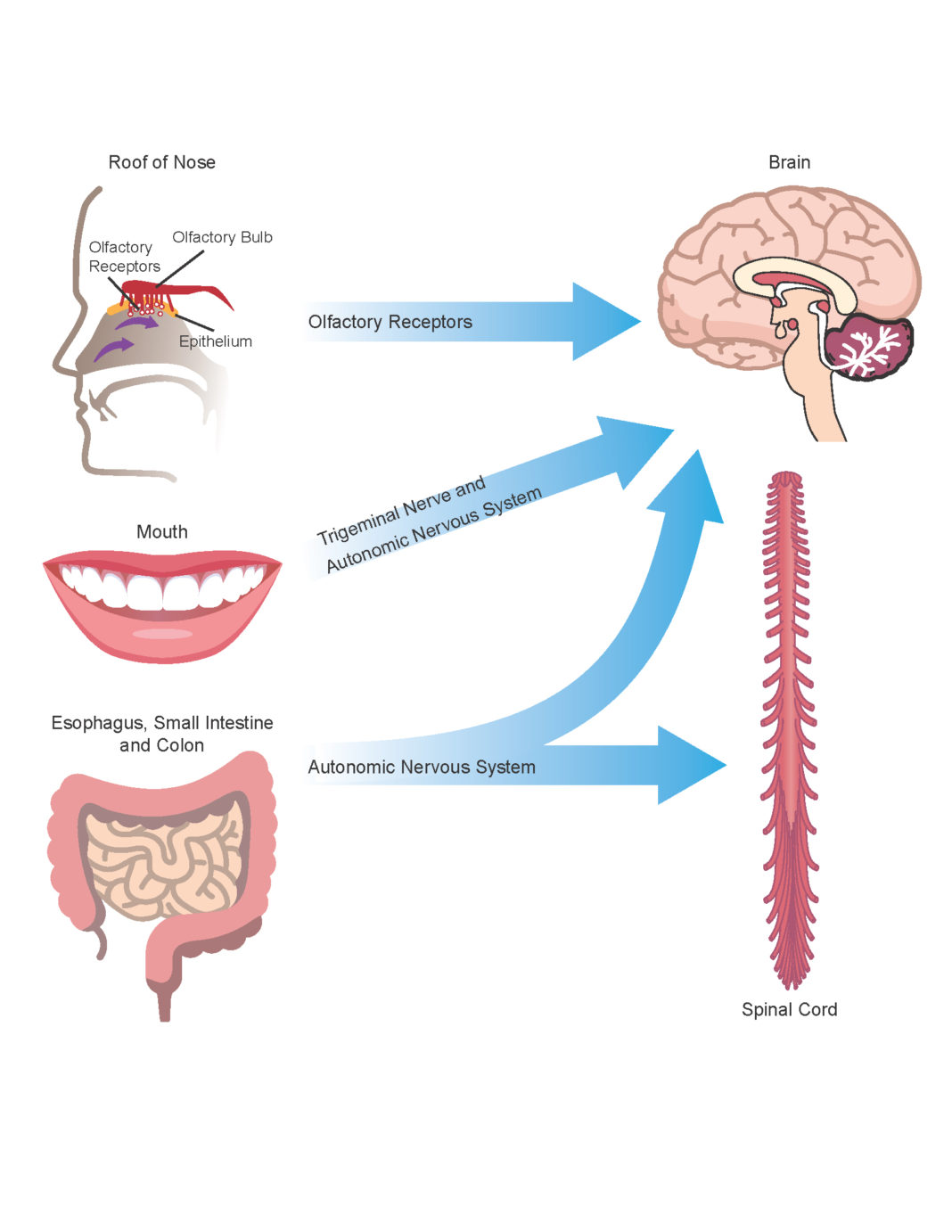
Research into the multitude of microbes that inhabit the human body has expanded considerably in recent years. Genomic analysis has begun to reveal the full diversity of bacteria, viruses, fungi, archaea and parasites living in and on the body, the majority of them in the gut. Even more recently, researchers have begun to explore how the proteins and other metabolites produced by microbes inhabiting the gut influence functions in other parts of the body, including the brain. However, we do not yet have a full understanding of how these systems work. The relationship between the microbiota and the brain has been called the “gut-brain axis.”
It is understood that the clumping of misfolded amyloid proteins, structures produced by neurons in the brain, are associated with neurodegeneration and conditions such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis (ALS).
“It is well known that patterns of amyloid misfolding of neuronal proteins are involved in age-related brain diseases. Recent studies suggest that similar protein structures produced by gut bacteria, referred to as bacterial amyloid, may be involved in the initiation of neurodegenerative processes in the brain,” Friedland said. “Bacterial amyloids are produced by a wide range of microbes that inhabit the GI tract, including the mouth.”
In research published in 2016 in Scientific Reports, Friedland and colleagues showed that when E. coli microbes in the gut of rats and worms (nematodes) produced misfolded amyloids, the amyloids produced in the animals’ brains and intestines also misfolded, a process called cross-seeding.
“Our work suggests that our commensal microbial partners make functional extracellular amyloid proteins, which interact with host proteins through cross-seeding of amyloid misfolding and trigger neuroinflammation in the brain,” Friedland said.
In today’s article, Friedland and Chapman also address other factors related to the microbiota and its products and how they influence neurodegenerative disorders.
- The microbiota modulates (enhances) immune processes throughout the body, including the central nervous system.
- The microbiota may induce oxidative toxicity (free radicals) and related inflammation that contributes to neurodegeneration.
- Metabolites produced by the microbiota may be either beneficial (health sustaining) or damaging (pathogenic).
- Host genetics influence microbiota populations, illustrating that the gut-brain axis is bidirectional.
Friedland believes further research in this area may lead to therapies for these neurodegenerative diseases, which are increasing in frequency and for which there are few effective treatments.
Chapman’s research is supported by the National Institutes of Health. Friedland’s work has been supported by The Michael J. Fox Foundation.
#WeAreUofL

























