Among researchers exploring the mechanisms of muscle growth and health, there have been certain conceptions about the role of the signaling protein, transforming growth factor-ß-activated kinase 1 (TAK1). Convention was that TAK1 is detrimental to muscle health since it activates pathways associated with muscle wasting.
“TAK1 is a very important molecule in the body and it is involved in the regulation of almost all cell types. It is implicated in many signaling processes and many physiological roles in the body,” said Ashok Kumar, PhD, a professor and distinguished university scholar in the University of Louisville Department of Anatomical Sciences and Neurobiology. “But the role of TAK1 in skeletal muscle was not known at all.”
Kumar and Sajedah M. Hindi, PhD, a postdoctoral fellow in the department, hypothesized that by removing TAK1, they could mitigate the negative effects of two downstream pathways associated with muscle wasting, NF-κB and p38 MAPK, with a single action. They and other members of the research team devised a series of cell culture and animal model experiments to determine if removal of TAK1 would preserve muscle mass and strength.
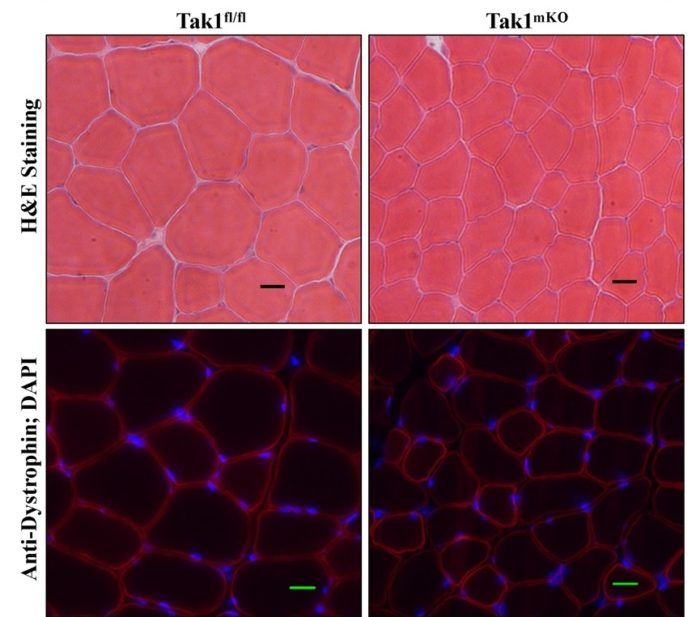
Their first clue to the significance of TAK1 was that mice genetically modified to remove TAK1 in skeletal muscle all died shortly after birth. Shifting their strategy, the researchers began working with adult mice. They found that in mature mice, instead of increasing muscle mass, reducing TAK1 resulted in severe muscle wasting, along with abnormalities in mitochondria and oxidative stress. These changes are consistent with those witnessed in muscle of individuals with amyotrophic lateral sclerosis (ALS), type II diabetes, cancer and aging.
“It did the opposite of what we were hoping it would do,” Hindi said. “In other tissues, having too much TAK1 has a bad effect. Knocking it down is actually positive. But in mature skeletal muscle, knocking TAK1 down had a negative effect.”
The research is detailed in TAK1 regulates skeletal muscle mass and mitochondrial function, published today in the journal JCI Insight, authored by Hindi, Kumar, Shizuka Uchida, PhD, associate professor and researcher in the UofL Cardiovascular Innovation Institute, Bradford Hill, PhD, associate professor and researcher in the UofL Diabetes and Obesity Center, and others at UofL.
This research reveals the essential role of TAK1 for the health of mature skeletal muscle, and adds to work by Kumar, Yuji Ogura, PhD, and Hindi, published in 2015 in Nature Communications, revealing that TAK1 is required for adult muscle cell proliferation and survival and for the regeneration of adult skeletal muscle upon injury. That research showed that when TAK1 is reduced, satellite stem cells do not vigorously self-renew and many eventually die. Alternately, when TAK1-regulated signaling is increased, the satellite cells prosper.
Kumar believes this understanding of the essential role of TAK1 in muscle health could lead to the development of therapies to preserve muscle mass in the elderly and in individuals with muscle-wasting diseases such as muscular dystrophy, cancer, type II diabetes and ALS.
“This is a very fundamental discovery that people had a misconception about this pathway. This protein is very important for muscle maintenance,” Kumar said. “The next question is whether this is a mechanism for loss of muscle mass in all these conditions. We have approaches now to put this protein back into the body. If we put it back in the muscle or we have some drugs that activate this molecule, can we improve the muscle mass, can we preserve the muscle mass?”






























