KEY POINTS:
- Breath analysis offers an option for primary screening and post-surgery monitoring of lung cancer patients.
- Certain carbonyl volatile organic compounds in exhaled breath indicate the presence of lung cancer.
- Researchers hope to get FDA approval for this new process.
CHICAGO — A single breath may be all it takes to identify the return of lung cancer after surgery, according to a study authored by University of Louisville Researchers and posted online today by The Annals of Thoracic Surgery.
Exhaled breath contains thousands of volatile organic compounds (VOCs) that vary in composition and pattern depending on a person’s health status. A subset of four VOCs—called carbonyl compounds because of their carbon base—have been discovered in the exhaled breath of lung cancer patients. Being able to identify this lung cancer “signature” through a simple breath test has emerged as one of the most promising ways to diagnose the disease. Now the test is being used to monitor for disease recurrence.
Erin M. Schumer, M.D., Victor van Berkel, M.D., Ph.D., and colleagues from the University of Louisville analyzed breath samples collected before and after surgery from 31 lung cancer patients and compared their carbonyl VOCs levels with samples from 187 healthy patients.
The researchers found a significant decrease in overall carbonyl VOC levels following surgery; in fact, three of the four carbonyl VOCs normalized after surgery, matching levels in the control group.
“The rapid normalization of almost all of the four compounds after surgery provides strong evidence that they are directly produced by the tumor environment,” Schumer said. “This study confirms that the technology is accurate.”
Lung cancer is the leading cause of cancer death. The American Cancer Society estimates that more than 224,000 Americans will be diagnosed with lung cancer this year, and more than 158,000 lung cancer patients will die—that translates to 433 lung cancer deaths per day in the United States.
Schumer said those grim statistics underscore the need for early detection, “We hope that breath analysis will allow us to diagnose patients with primary or recurrent lung cancer long before they suffer from symptoms, when we have more options for treating them, giving them the best chance for cure.”
Currently, lung cancer patients are followed after surgery with chest computed tomography (CT) scans, which can be inconvenient, expensive, and expose the patient to radiation. “We hope that the breath analysis can serve as the primary screening tool for cancer recurrence and a CT scan ordered only if the breath test suggests that there has been a change,” van Berkel said.
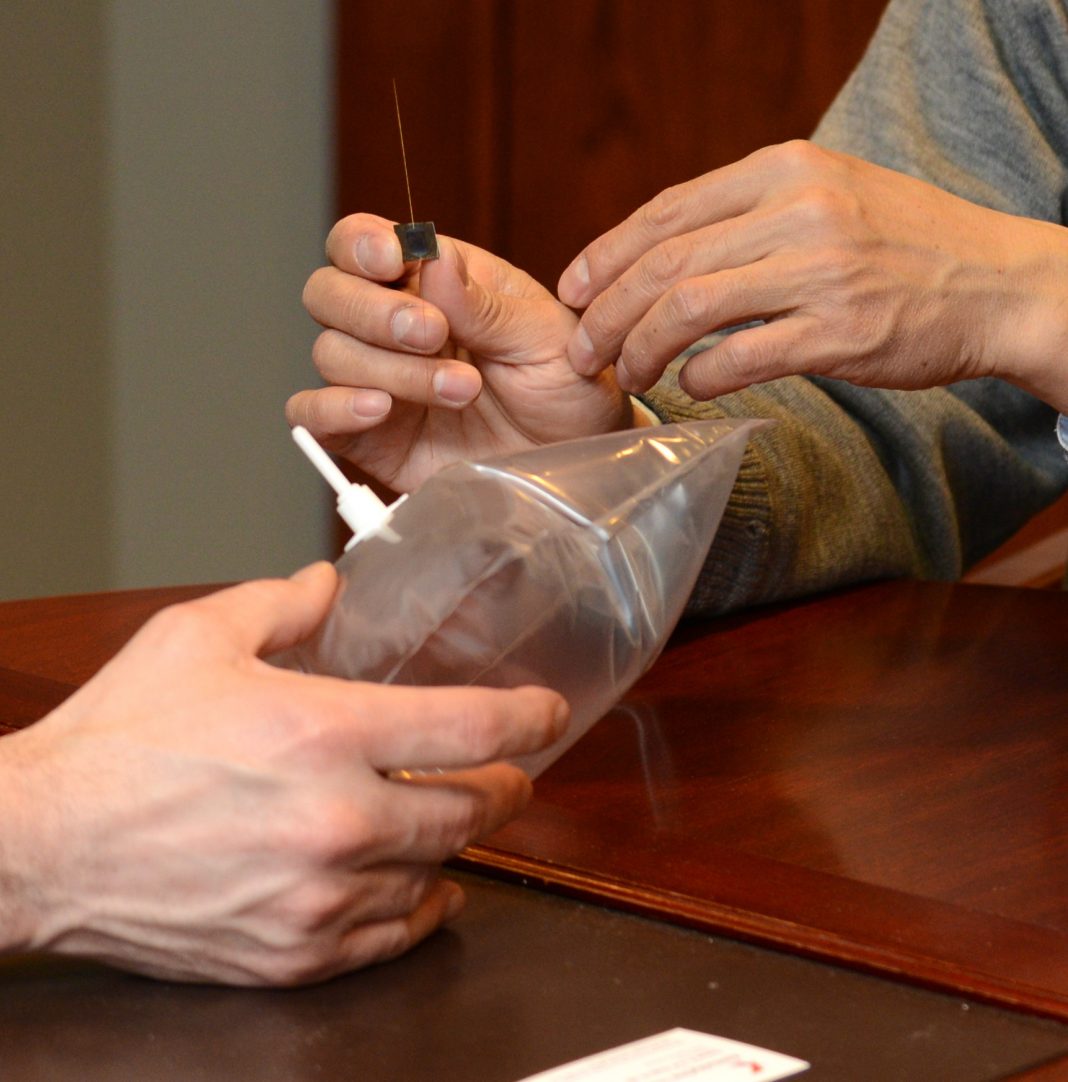
How the breath test works
The process of breath analysis is relatively simple. The patient blows a single breath into a specialized balloon. The balloon is then connected to a pump that pulls the breath over a small microchip that is smaller in size than a quarter, trapping the chemicals. The microchip is sent to the lab, where the chemicals are analyzed within hours. Breath collection can be performed in the doctor’s office.
The pump is reusable; the balloon, microchip and lab test together cost around $20, all supporting the increasing acceptance of breath tests as a cost-effective, easy-to-perform, non-invasive and rapid option for the diagnosis of lung cancer.
“The great potential with breath analysis is detecting lung cancer at any point, both as a primary screening tool and to follow patients after disease has been treated,” van Berkel said. “The technology is pretty robust. Our next step is getting approval from the FDA.”























